What is the evidence to support the 2-metre social distancing rule to reduce COVID-19 transmission?
June 22, 2020
Zeshan Qureshi1, Nicholas Jones2, Robert Temple3, Jessica PJ Larwood4, Trisha Greenhalgh,2 Lydia Bourouiba5
1 St Thomas’ Hospital, London, UK
2 Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK
3 Somerville College, University of Oxford, UK
4 St John’s College, University of Oxford, UK
5 Massachusetts Institute of Technology, Cambridge, MA, USA
Correspondence to zeshan.qureshi@nhs.net or lbouro@mit.edu
Lay Summary by Mandy Payne, Health Watch
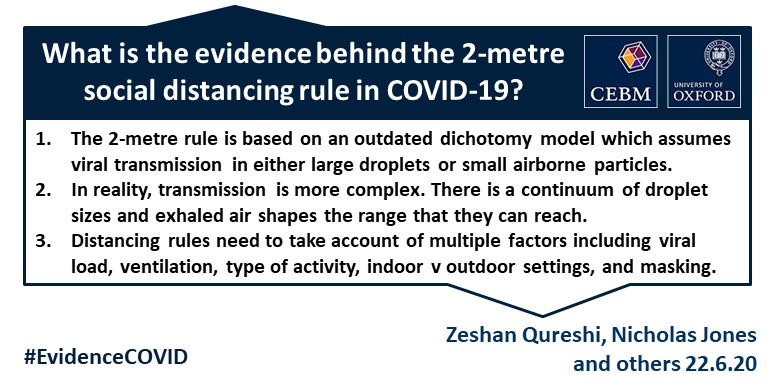
VERDICT
- The 2-metre social distancing rule assumes that the dominant routes of transmission of SARS-CoV-2 are via respiratory large droplets falling on others or surfaces.
- A one-size-fits-all 2-metre social distancing rule is not consistent with the underlying science of exhalations and indoor air. Such rules are based on an over-simplistic picture of viral transfer, which assume a clear dichotomy between large droplets and small airborne droplets emitted in isolation without accounting for the exhaled air. The reality involves a continuum of droplet sizes and an important role of the exhaled air that carries them.
- Smaller airborne droplets laden with SARS-CoV-2 may spread up to 8 metres concentrated in exhaled air from infected individuals, even without background ventilation or airflow. Whilst there is limited direct evidence that live SARS-CoV-2 is significantly spread via this route, there is no direct evidence that it is not spread this way.
- The risk of SARS-CoV-2 transmission falls as physical distance between people increases, so relaxing the distancing rules, particularly for indoor settings, might therefore risk an increase in infection rates. In some settings, even 2 metres may be too close.
- Safe transmission mitigation measures depend on multiple factors related to both the individual and the environment, including viral load, duration of exposure, number of individuals, indoor versus outdoor settings, level of ventilation and whether face coverings are worn.
- Social distancing should be adapted and used alongside other strategies to reduce transmission, such as air hygiene, involving in part maximizing and adapting ventilation to specific indoor spaces, effective hand washing, regular surface cleaning, face coverings where appropriate and prompt isolation of affected individuals.
BACKGROUND
It is well established that respiratory viruses can be transmitted via viral-laden mucosalivary respiratory droplets. These are expelled during exhalation, speech, and more forcefully during coughing and sneezing (categorised as ‘violent respiratory events’).1 Traditionally, transmission of respiratory disease was thought to occur via one of two distinct routes (and this classification is still being used): droplet route for large droplets and aerosol or airborne route for small droplets. The former assumes that large droplets fall on surfaces or on others, and contributes to surface contamination. The latter assumes inhalation of pathogen-bearing droplets invisible to the naked eye, and typically smaller than 5-10 microns in diameter. Based on this dichotomy framework, SARS-CoV-2 virus is currently thought to be spread by ‘contact and droplet’ transmission,2 though scientists are debating the possibility of airborne transmission.
Public health infection control measures are based largely on this classification, with different interventions recommended for large droplets and airborne droplets. Whilst some level of personal protective equipment (PPE) and hand hygiene are required in all cases, additional infection-control measures are recommended for airborne transmission in high-risk settings, such as the use of respirators and negative pressure airborne individual isolation rooms.3 A parallel rapid review in this series, covering which clinical procedures are classed as aerosol generating, is due to report shortly. Maintaining physical distance from other people may be effective at reducing transmission of both droplet and airborne spread of communicable disease. So-called ‘social distancing rules’ have been implemented in many countries to reduce the risk of COVID-19 transmission, with early implementation of social distancing linked to decreased incidence.4 5
Whilst this conceptual framework of droplet size can be useful, the dichotomy between large droplets and small airborne particles emitted in isolation is an oversimplification. In fact, respiratory infections are transmitted via a continuum of droplet sizes embedded in a cloud of exhaled air containing those visible to the naked eye (millimeters), to invisible ones, in the micron scale. This droplet size continuum and the cloud that carries them have significant implications for mode of transmission. Social distancing rules are based on estimations of risk of droplet transmission in relation to isolated large droplet emission only. Therefore, if SARS-CoV-2 were only transmitted in large isolated droplets, this would imply shorter physical distancing measures would be sufficient to reduce risk.
High rates of secondary infection have been reported among household members and close contacts of people with COVID-19, who are likely to get within 1-2 metres.6-9 The World Health Organization (WHO) have suggested adopting a 1 metre social distancing policy, based primarily on the assumption that SARS-CoV-2 is transmitted in large isolated droplets.2 Individual countries have subsequently set their own social distancing policies, with some countries (such as Spain and Canada) implementing a required 2-metre distance rule. The UK government’s recommendation is currently 2 metres but this is under review at the time of writing.10 It is important that this distance is set appropriately and flexibly. Too short a distance imposed too rigidly risks avoidable transmission, whereas too long is unnecessarily disruptive to society.
This review aims to identify the evidence behind the 2-metre social distancing rule in the context of the still used large vs. small (droplet vs airborne) droplet size dichotomy in route of transmission. It will look specifically at transmission risk in relation to physical distance and air sampling studies around COVID-19 patients, but also wider evidence about whether airborne transmission should be considered a possible mechanism of SARS-CoV-2 spread.
What is the evidence for how far respiratory droplets of different size travel?
The origin of the 1-2 metre social distancing rule for communicable disease significantly predates COVID-19. A 1942 study by Jennison, referenced as early evidence for use of the 1-2 metre limit, used high-speed exposure still photography to detect atomized secretions and found that the majority of droplets were expelled within 1 metre.11 However, the technology was insufficient to capture smaller droplets and the chosen observation field for imaging was set at 1-2 metres, meaning longer distance of droplet spread was not part of the study. Over time, limited epidemiologic and simulated studies of selected infections such as rhinovirus and meningococcal disease have generated some evidence to support 1-2 metres of social distancing.12-14 However, these earlier studies also used methods that lack accuracy compared to current standards, particularly with respect to air sampling. For example, Duguid et al used a method involving impact on glass slides and found no droplets less than 5µm were captured during coughing onto exposed slides held within 6 inches of the face,15 but this result is not consistent with more recent data with modern air sampling methods of detecting droplet spread.
In 2020, Bahl et al conducted a systematic review looking at reported horizontal distance travelled by respiratory droplets.11 This is useful as a proxy measure for how far associated viral particles might travel, and therefore risk of infection transmission in relation to distance. Eight of 10 studies demonstrated a horizontal trajectory greater than 2 metres for particles up to 60µm. In one of the only studies using direct measurements involving human subjects in addition to modelling, Bourouiba et al took and analysed direct high-speed imaging of human sneezes and coughs. They noted the importance of the exhalation gas cloud in transporting all drops forward. They showed that although the largest droplets visible with the naked eye (order of millimeters) rapidly settled within 1-2 metres, the other droplets could be observed in air 6-8 metres away.16-18 This shows the potential for viral particles to be projected extensively in a room within seconds of their emission.
Multiple studies have suggested possible spread beyond 2 metres from an index patient in the recent SARS, MERS and avian flu outbreaks.19 20 For example, Wong et al reported on transmission in relation to a SARS-CoV-1 outbreak amongst medical students exposed to a single patient in hospital.21 Of 27 students who entered the patient’s cubicle (used as a proxy marker for being within 1 metre), 10 developed the disease. However, 1/20 who denied entering the cubicle, and 4/18 who couldn’t recall if they had entered the cubicle, also developed the disease, which could suggest either secondary transmission or possible transmission over longer distances.
What factors influence the distance respiratory droplets spread?
Given droplet sizes are on a continuum rather than binary large or small, the distance droplets travel will also be over a continuous range and influenced by a number of factors, other than droplet size alone. For example, recent research by Bourouiba et al has shown by direct quantification, modelling, and validation on human subjects that ‘violent respiratory events’, e.g. coughing and sneezing, generate a warm, moist and turbulent gas cloud with forward momentum. This can significantly extend the distance travelled by a virus in a room within seconds and independent of background ventilation or airflow. This phenomenon and other developments in our understanding of exhalation dynamics are not accounted for in the earlier modelling studies of droplet transmissions on which the current dichotomous classification of large versus small droplets is based.16
Even volume of speech may impact on droplet spread and subsequent risk of transmission, making the process of predicting mode of transmission problematic.22 Clusters of coronavirus have occurred during prolonged ‘violent exhalation events’ such as singing or fitness dance classes in confined locations.23 24 For example, Hamner et al report that a two and a half hour choir rehearsal with one symptomatic person led to 32 confirmed and 20 probable COVID-19 cases among the 61 singers, even though all singers avoided any direct physical contact.24
Viral shedding (higher with coughing/sneezing) and factors related to airflow in indoor environments, such as ventilation, may increase droplet spread. Nishiura et al used contact tracing to collect secondary transmission data from 110 index cases with COVID-19 across 11 clusters in Japan. All clusters were linked to indoor spaces, including fitness gyms and a restaurant boat. The authors report that the odds of transmission in an enclosed environment were 18.7-fold higher than in an outdoor environment.25 Other indoor case clusters have been reported in gyms, churches, hospitals and elderly care settings.26 27 Conversely, facemasks may help limit airborne droplet transmission.28 Such case reports require further investigation but imply environmental factors are important in addition to physical distance in determining risk of transmission.
This indirect evidence illustrates that safe social distancing limits differ widely between settings, with outdoor environments likely associated with lower risk of transmission at a given distance. Staggered social distancing rules alongside other public health interventions may be required to recognise the importance of the environmental context in determining transmission risk.
What is the evidence that there is live virus in these different sized droplets at different distances?
Respiratory virus RNA is detectable in both large droplets that settle on surfaces and airborne droplets after breathing or ‘violent respiratory events’.29 Both animal and human volunteer influenza inoculation studies have suggested that deep inhalation (which occurs to a far greater extent with aerosols than droplets) may result in similar or even greater infection rates compared to intranasal large droplet inoculation.30-32 However, the relative contribution of the airborne route to actual transmission remains a subject of debate.17 33 34 This may in part be due to variation in host, viral, and environmental factors for each interaction.35 Factors suggested include virus concentration in respiratory fluid, levels of pollution or particulate matter in the air, humidity, temperature, indoor versus outdoor environments, symptomatic versus asymptomatic hosts, and an individual’s baseline susceptibility to infection.
SARS-CoV-2 is present in sputum.36 Van Doremalen et al, analysed SARS-CoV-2 across 10 experimental conditions in five environments and showed that the virus is also stable in air for at least 3 hours,9 with others suggesting it may be stable for up to 16 hours.37 Reports from cluster outbreaks such as the choir practice provide indirect evidence there is live SARS-CoV-2 in respiratory droplets.24 There is also additional indirect evidence suggesting potential airborne transmission. One study showed that SARS-CoV-2 is deposited deep in the airways of hospitalized patients.36 This tends to be associated with an airborne route of transmission, whereas diseases of the upper respiratory tract tend to be associated with rapidly settling droplets and surface contamination. Additionally, asymptomatic spread of coronavirus has been confirmed in several studies,38-40 which is consistent with airborne transmission as larger visible droplets are disproportionately emitted in coughing and sneezing.41 42
What is the evidence that 2 metres is an adequate distance to reduce SARS-CoV-2 transmission?
Search strategy
To determine what evidence there is to support the 2-metre social distancing rule specific to SARS-CoV-2, we conducted a search of PubMed, MedRxiv, LitCOVID and Google scholar, using the terms listed in the appendix. We included studies reporting transmission risk in relation to distance across any setting. We also included studies reporting airborne sampling for SARS-CoV-2, as we felt these might provide information as to the potential spread of the virus in relation to distance. The search was run from inception to 17th June 2020. From 3,549 papers identified in the search and an additional 58 studies through forward and backward citation checking, we reviewed 120 full texts. From these we included 25 studies directly reporting SARS-CoV-2 transmission risk in relation to physical distance. We also included additional texts in the review that report distances in droplet spread not specific to SARS-CoV-2, though these were not the focus of the search.
Overview of studies
Aside from one systematic review across settings, we categorise results into community (n=10) and hospital-based studies (14), given disease prevalence and severity are likely to differ significantly. Community settings included cruise ships (2), household contacts (3), a restaurant (1), a shopping mall (1) a medical conference (1) a multi-storey mixed commercial and residential-use building (1), and a multi-site study (1). In addition to the systematic review (1), individual studies were based in China (10), the USA (4), Singapore (2), Germany (2), the United Kingdom (1), South Korea (1), Taiwan (1), Thailand (1) and two aboard cruise ships. None were specifically set outdoors or in schools. Additionally, although the demographics of several of the studies are unclear, none look specifically at children or babies.
Quality assessment
Most of the included studies had not yet undergone peer review and many were felt to be at risk of bias due to small numbers of study participants, and methods lacking transparency or reproducibility. There was heterogeneity across the studies in terms of methods, population and research question. Many studies were of retrospective design, and hence at risk of recall bias in terms of distance to infected individual and selection bias in terms of identifying recent contacts, particularly those that relied on contact tracing alone. Confounding variables, such as disease severity, time since symptom onset and contact with other individuals, were rarely reported. Publication bias was not formally assessed but must be considered, particularly in the reporting of clusters of cases. All of these factors may be important in understanding the variation in reported results.
In the air particle studies, only two incorporated the capacity to directly measure the infectivity of coronavirus, rather than just the presence of viral RNA in the air. Heterogeneity specific to these studies included differences in hospital cleaning and ventilation, variability in air sample volume and sample handling for viability test. These studies also tended to include insufficient discussion of systematic calibrations for sampling instrumentation or sensitivity of viability to sample collection and handling methodologies. This makes it difficult to interpret the results objectively and compare results between sampling studies.
A recent systematic review and meta-analysis published in The Lancet assessed the evidence for reducing risk of SARS-CoV-2 transmission.43 They aimed to investigate optimum physical distancing for avoiding person-to-person coronavirus transmission, and to assess the impact of face masks and eye protection on preventing transmission. Studies of any design in any setting were included if they reported on these outcomes among WHO-defined confirmed or probable COVID-19, SARS, or MERS, published before 3rd May 2020.
However, this review draws largely on evidence from SARS and MERS with only seven studies included of COVID-19, five of which were non-peer reviewed pre-prints and one a correspondence piece. There was significant heterogeneity between the included studies in terms of setting, indoor and air conditions, degree of physical distancing and identification of cases, which makes it difficult to draw conclusions as to the safety of respective distances. As with the other observational studies we include in our review, there is risk of recall bias in terms of people remembering how close they were to contacts and selection bias in terms of regular contacts of infected cases being more likely to be included. Such limitations mean there is low or very low certainty in these summary findings and thus no methodological ability to distinguish between routes of transmission in an indoor space.
SARS-CoV-2 transmission risk across all settings
In their Lancet systematic review, Chu et al reported that closer contact distance is associated with an increased transmission risk of SARS-CoV-2, across study settings. In a sub-group analysis focused on SARS-CoV-2, the relative risk of developing COVID-19 among people who had been in “close” compared to “distant” contact with an infected case was 0.15 (95%CI 0.03 to 0.73). However, the threshold for ‘further distance’ varied from anything over direct contact in some studies to 2 metres in others, meaning a close contact in one could be a distant contact in another. Chu et al estimated this key physical distance for some studies where it had not been explicitly reported. Furthermore, there was no real accounting for other variables affecting transmission risk beyond just social distancing, and that may explain some of the variability between studies. However, in a meta-regression of change in relative risk of developing SARS-CoV-1, SARS-CoV-2 or MERS in relating to increasing distance, the risk of being infected is estimated to be 13% for those within 1 metre, but only 3% beyond that distance. The authors go on to conclude that there is good evidence to support physical distancing of at least 1 metre, but 2 metres may be more effective, whilst acknowledging a range of factors influence transmission risk. This analysis assumes transmission risk in relation to distance is fixed and does take account of important variables such as duration of exposure or the indoor air and environment. The meta-analysis also found some evidence to support facemasks (aOR 0·15, 95%CI 0·07 to 0·34 with stronger associations with N95 or similar respirators) and eye protection (aOR 0·22, 95% CI 0·12 to 0·39) for reducing transmission of coronavirus. Eye protection benefits would be consistent with the airborne route of transmission, given that smaller particles are likely to remain present in the air and be absorbed via the conjunctival surface.44
SARS-CoV-2 transmission risk in community studies
The ten community studies in our sample included five that retrospectively analysed the impact of physical distance on COVID-19 outbreaks, three contact tracing studies and two studies of air sampling around COVID-19. Five studies reported clusters of cases among individuals who had prolonged exposure to an infected individual within a confined space,38 45-48 and a single modelling study also suggests this may be important for transmission.49 Spouses and close household contacts also appeared to be at increased risk, compared to community contacts,50 51 though interestingly air sampling studies in the houses of people with COVID-19 were negative.52 Although some studies suggested possible transmissions at distances in excess of 2 metres,47 53 there wasn’t sufficient description to rule out transmission via close or direct contact in any of the studies. We describe these studies in more detail below.
Retrospective analyses of COVID-19 outbreaks
Li et al reviewed study analysed an outbreak of 10 new cases of COVID-19, all of whom were infected in a single sitting at a restaurant in Guangzhou, China.47 The 10 cases across three families all sat in adjoining tables at one end of the restaurant. Transmission is believed to have taken place between these individuals despite no significant close contact between the involved families on video analysis. Distances between index and patrons infected were noted to be up to 4.6 metres. The pattern of transmission was compatible with flow patterns of indoor ventilation in the restaurant, which they were sitting beneath. Transmission via touch cannot be excluded but there was a specific pattern of infection along the downstream flow line from the ventilation source and no cases were reported from other tables within the restaurant. Both the pattern of transmission, and the distance of separation support airborne transmission.
Hijnen et al noted the likely spread of COVID-19 at a dermatology meeting of 14 people, of which 12 subsequently tested positive including the index case.38 At the meeting, individuals sat 2.6 metres away from the index individual over a two-day meeting. They also shook hands and shared taxis, giving opportunity for closer contact.
Cai et al analysed a 17-person outbreak of COVID-19 at a shopping mall in China.53 They found that several individuals who contracted the disease worked on different floors to the index case, raising the possibility of longer distance transmission through air. This might also be explained by shared routes to work, elevators, spread from asymptomatic individuals, congregation of staff, or contaminated customers moving between floors.
Park et al investigated an outbreak of COVID-19 cases at a single 19 storey building, containing a mix of residential and commercial units.45 Contact tracing was initiated after the first index case was detected, leading to 97 confirmed cases of 1,143 tested, 89 (91.7%) of whom were symptomatic. Most of these positive cases (n=94, (96.9%)) worked in an 11th floor call centre, which had 216 employees in total. Although the index case did not visit this call centre or floor, the second case-patient was an employee at the call centre. There was a secondary attack rate of 34 (16.2%) in the households of the positive cases.
Xu et al published an analysis (not yet peer reviewed) of the COVD-19 outbreak aboard the Diamond Princess Cruise Ship, where 696 people were infected of the 3711 aboard.46 They included 197 initial symptomatic cases as well as an additional 146 subsequent passenger cases, 129 of whom had been in ‘close contact’ with one of the original infected individuals, defined as staying within the same state room, and 17 who developed COVID-19 but had not been in close contact with an index case.46 Their analysis suggests these 17 people were infected before quarantine measures were introduced, suggesting there was no distant spread following quarantine. This was a retrospective model, and provides limited evidence as to safe distancing limits and routes of transmission in indoor spaces.
Air sampling studies
The second cruise ship study, also non-peer reviewed, aimed to establish whether contaminated environmental surfaces were important in determining risk of SARS-CoV-2 transmission.54 The study team directly sampled air in cabins of those with and without COVID-19, finding no evidence of coronavirus across 14 air samples, but evidence of it on surfaces, such as bed pillows and around the toilet, based on positive RNA results. However, samples were taken up to 17 days after the residents had left their cabins and the negative results could be a reflection of this long lag time before testing. Döhla et al analysed air samples from 21 households under lockdown where a family member had tested positive for COVID-19 and reported all samples were negative.52 Timing in relation to cleaning and distance from the infected person was unclear.
Contact tracing studies
Doung-ngern et al conducted a retrospective case-control study in Thailand using contact tracing to identify 1,050 asymptomatic individuals who had been in close contact with 18 primary index patients with COVID-19.48 Close contacts included household members or non-household contacts who had been within 1 metre of the index patient for longer than 5 minutes. Most people contacted the index cases at a boxing match (n=645), night club (n=374) or at a state enterprise office (n=31). Of these initially asymptomatic people, 211 (20%) were later diagnosed with COVID-19. People who did not come within a 1 metre distance of the index case were at significantly lower risk of developing COVID-19 (adjusted odds ratio 0.15, 95%CI 0.04 to 0.63), whilst facemasks and handwashing were also associated with reduced risk.
In a prospective study among a Taiwanese population, Cheng et al included 32 confirmed cases of COVID-19 and their 1,043 recent contacts to determine the secondary attack rate (i.e., spread of disease onto other close contacts) and determinants of transmission. There were 15 cases identified as secondary attacks in close contacts, defined as people who had spent 15 minutes or more in face-to-face contact with the infected individual. There was no statistically significant difference in secondary attack rates between household contacts (13.9%, 95%CI 4.7 to 29.5%) and family contacts not living in the same household (8.5%, 95%CI 2.4 to 20.3%).51 There were no secondary cases reported amongst more distant contacts, including healthcare workers.
Burke et al identified nine cases with COVID-19 early in the outbreak in the USA, and 404 of their close contacts who agreed to participate in monitoring. Of these close contacts, 159 had 1 or more respiratory swab taken. There were only 2 cases of secondary transmission, both among spouses, giving a secondary attack rate of 13% (95%CI 4 to 38%) among the 15 household contacts. Exposure to the infected individuals was longer for these two spouses than the 13 individuals who did not develop COVID-19.50 None of the healthcare professional or community contacts developed swab-positive COVID-19, though many had suspected symptoms, potentially calling into question the accuracy of the tests used.
SARS-CoV-2 transmission risk in hospital settings
Of the fourteen hospital studies, nine analysed air samples around patients with confirmed COVID-19 as a proxy measure for possible airborne spread (Table 1). Seven of these studies reported positive airborne samples for SARS-CoV-2, including two at distances of 2 metres or greater from the source patient. Although the presence of SARS-CoV-2 in airborne particles does not confirm transmission at distance, it does demonstrate the extent to which viral-laden droplets may travel in air from an infected individual, consistent with what is known about high momentum cloud exhalation range55 and not consistent with the ballistic large droplet route, in which the all contaminated droplets would fall on surfaces within the 1-2 m vicinity of the patient.
Air sampling studies
Zhou et al investigated airborne spread of SARS-CoV-2 at a multi-site London Hospital.56 Viral RNA was detected on 14/31 (38.7%) air samples and 114/218 (52.3%) of surface samples, with more frequent positive results in areas occupied by patients with COVID-19. No virus was deemed viable (i.e. capable of being replicated) from any site. However, this may have been due to the time lag between droplets deposition and culture, and small air sample volumes. Liu et al found positive SARS-CoV-2 air samples across 60% of tested sites in two dedicated COVID-19 hospitals.57 Chia et al conducted air sampling in the rooms of three patients with confirmed COVID-19. Positive results were obtained from the two patients who were on day 5 of symptoms but not the patient who was on day 9.58 Ma et al analysed air and surface samples of local indoor environments of COVID-19 recruited subjects in addition to their exhaled breath condensate. They found positive surface (5.4% out of n = 242) and air samples (3.8% out of n = 26) in addition to 103-105 viral RNA copies/min in exhaled breath associated with a positive sampling rate of 16.7% out of n = 30 samples.59 Santarpia et al reported positive samples collected from air samplers worn by sampling personnel in the vicinity of patients and at fixed distance locations from the patient, even when the patient did not cough. Additionally, they reported positive air samples from fixed collectors located at least about 2 meters away (6 feet).60 They also assessed viral viability, reporting signs of viability via viral propagation and using various indicators of viral replication. Indication of replication competence was reported for windowsill and hallway samples despite small volume sample preventing a full replication study. They noted that the small volume of recovery was problematic.60 Indeed, although there were no viable virus samples in most other air samples across the studies, there were also no viable virus samples on surface samples either. Hence, lack of viability in these studies cannot be used to discriminate between routes of transmission at this stage.
In contrast, Ding et al analysed 46 air samples from a hospital in Nanjing, including from isolation rooms of people with COVID-19 and found only one weakly positive result, which came from a ward corridor. However, they found that the exhaled condensate and two expired air samples from patients were also negative.61 It is also unclear whether the isolation rooms were occupied at the time of sampling, which might explain these negative environmental sampling results. Ong et al. also reported negative air samples taken in the vicinity of three patients with COVID-19, but positive samples in the room air exhaust outlets, consistent with the airborne route and suspension and clearance of airborne virus-laden particles by ventilation.62 Viral particles in air-vents is not consistent with the hypothesis that the virus would only be contained in large drops deposited ballistically on surfaces in the 1-2 m patient vicinity. Wu et al also detected no positive air samples among 44 collected on a medical ward caring for people with COVID-19.63 However they discussed the limitation of their findings with the need for larger air sampling volume.
Only two sampling studies explicitly commented on distance. Guo et al reported airborne samples positive for SARS-CoV-2 up to 4 metres from the patient,64 and Sanatarpia up to at least 2 metres.60
Additional studies
Wong et al in Hong Kong undertook contact tracing around a patient with COVID-19, receiving oxygen and treated for pneumonia.65 Although 52 individuals came into contact with the index case and subsequently developed fever or respiratory symptoms, all tested negative for SARS-CoV-2. No explanation is given for the high rate of symptoms in these contacts and the results must be considered with caution.
Heinzerling et al undertook a study in California, reporting that 3/33 healthcare professionals who came within six feet of an index patient with COVID-19 later developed the infection and tested positive.66 Of these three, two had frequent direct contact with the index patient, including during aerosol generating procedures, but wore no facemask, eye protection or gown. The third staff member wore a facemask and gloves most of the time, but did not wear eye protection.
Bai et al reported 12/42 healthcare professionals in a hospital in Wuhan who had contact with either the index patient with COVID-19 or an affected colleague (distance undefined) developed the infection, compared to 0/76 colleagues without such contact.67 It is unclear to what extent each group complied with PPE advice. Burke et al investigated 126 healthcare professionals who had contact with nine patients with COVID-19. None developed COVID-19, even though 76 provided direct patient care, of which only 43% reported using appropriate PPE.50 Cheng et al studied 301 healthcare professionals exposed to 32 confirmed COVID-19 patients, defined as being within 2 metres without appropriate PPE, yet none tested positive for COVID-19.
Table 1. Summary of evidence from hospital-based studies looking at airborne transmission of SARS-CoV-2
| Study |
Year |
Setting |
COVID-19 Patients |
Air Samples |
Air Sample Volume |
Method of detection |
Peer Review |
Viability Test (can the virus replicate) |
SARS-CoV-2 Positive Air Samples |
Possible evidence for airborne transmission |
Comments |
| Liu57 |
2020 |
Wuhan, China |
Unknown, though the two hospitals were exclusively used for people with COVID-19 during outbreak |
30 sites across two hospitals including public sites |
Total sampling air volumes: 1.5 m³ to 8.9 m³, 5L/min rate.
Sampled between 5 hours and 7 days |
PCR |
Yes |
No |
21 positive samples across 35 sites (60%), though 4 sites were positive at first round of sampling and negative at second |
Yes |
Distance of air samples from patients unclear |
| Guo64 |
2020 |
Wuhan, China |
39 |
40 ICU samples and 16 general ward samples |
300l/min (30 minutes) |
PCR |
No |
No |
In ICU, 5/14 samples near the patient, 8/18 approximately 2.5 metres from the patient, and 1/8 samples approximately 4 metres from the patient. In general ward 2/11 positive near the patient and 0/5 at 2.5 metres distance. |
Yes |
Unclear why higher percentage of positive results at 2.5 metres from the patients compared to immediately by the patients. |
| Santarpia60 |
2020 |
Nebraska Medical Centre, USA |
11 rooms with 13 COVID-19 patients and hallways |
31 |
50l/min (15 minutes) |
PCR |
No |
Yes |
63.2% (including 2/3 where the air sampling was at least six feet (about 2 metres) from the patient and 58.3% in hallways. All non-viable. |
Yes |
Distance of air sample from the patient was not uniformly recorded for any samples other than the three reported. Indication of viral replication competence in samples identified, with limitation in volume sampled discussed as problematic. |
| Ding61 |
2020 |
Nanjing, China |
10 |
46 |
Multiple groups:
a) 10l/min
(30 min)
b) 50l/min (20 mins)
c) 500l/m (2 min)
d) 500l/m (20 min)
e) 14l/min (30 min) |
PCR |
No |
No |
2% i.e. 1 sample, which was weakly positive |
Yes |
The significance of weakly positive is unclear. Distance of air samples from patients unclear. |
| Chia58 |
2020 |
Singapore |
3 rooms |
18 |
3.5l/min (4 hours) |
PCR |
No |
No |
Positive samples in 2 of 3 rooms |
Yes |
Distance of air samples from patients unclear |
| Ong62 |
2020 |
Singapore |
3 |
Unclear |
5/l min (4 hours) |
PCR |
Yes |
No |
All airborne samples were negative. However, positive swabs were collected from 13/15 room sites, including 2/3 at the air outlet fan. |
Yes given positive samples at air outlet fans, although all air samples were negative |
Inconsistent methodology between samples reported. Majority of samples collected immediately after room cleaning
Distance of air samples from patient unclear |
| Zhou56 |
2020 |
London, UK |
7 Clinical areas and 1 public area of the hospital |
31 |
100L/min (10 mins) |
PCR |
No |
Yes |
38.7% suspected positive (at least one of two samples positive), 6.4% positive (both samples positive). All 8 areas produced at least one positive. All non-viable |
Yes |
Short sampling time.
Distances from patients unclear.
Positive samples more common in areas where COVID-19 patients were being cared for |
| Wu63 |
2020 |
Wuhan No.7 Hospital, China |
17 hospital areas including intensive care |
44 |
As per “Hygiene Standards for Disinfection in Hospital” in China |
PCR |
Yes |
No |
0% |
No |
Small air sample volumes acknowledged as a problem
Distance of air sample from patients unclear |
| Ma59 |
2020 |
Beijing, China. (Two hospitals and multiple quarantine hotel rooms) |
Samples taken from areas associated with 35 recruited COVID-19 patients. Hospital and quarantine hotel environments including corridors, hotel rooms, ICU emergency room and CT room and clinical observation room samples. |
26 |
15L/min in enclosed environments
400L/min in corridors |
PCR |
No |
No |
3.8% |
Yes |
Also sampled exhaled breath of 30 patients, 16.7% positive. Distance of air samples from patient unclear. Rooms well ventilated (open windows or negative pressure systems) |
CONCLUSIONS
- The longstanding dichotomy of large droplet versus small airborne droplet transmission is outdated and SARS-CoV-2 may be present and stable in a range of droplet sizes, which will travel across a range of distances, including some beyond 2 metres.
- The majority of existing evidence specific to SARS-CoV-2 is observational and non-peer-reviewed, with significant heterogeneity in terms of populations, study settings, sample collection methods and primary outcome. Determining the relative risk of SARS-CoV-2 at different distances is therefore difficult from such studies.
- Evidence from community studies suggest prolonged exposure in an enclosed space, with unknown information about distancing, may be linked to clusters of cases, particularly in the context of activities such as choirs, sports events or fitness gyms.
- Increasing physical distance is associated with decreasing risk, so easing restrictions from 2 to 1 metre may result in a significant increase in risk if other measures are not taken.
- Other factors such as duration of time spent with others in an indoor space, e.g. at work in a confined office and the indoor air conditions are as important to account for in the estimation and mitigation of risk.
- Single thresholds for social distancing, such as the current 2-metre rule, over-simplify what is a complex transmission risk that is multifactorial. Social distancing is not a magic bullet to eliminate risk. A graded approach to physical distancing that reflects the individual setting, the indoor space and air condition, and other protective factors may be the best approach to reduce risk.
- Other important factors to take account when considering safe social distancing (which were beyond the scope of this review to cover in depth) include host viral load, duration of exposure, number of infected individuals, indoor versus outdoor settings, air ventilation, wearing of PPE including facemasks, effectiveness and type of cleaning measures, individual susceptibility to infection, and activities that project airborne particles over greater distances in exhaled gas clouds, such as singing, coughing or heavy breathing.
- Social distancing should therefore be used in combination with other strategies to reduce transmission risk, including hand washing, regular surface cleaning, PPE and face coverings where appropriate, strategies of air hygiene, and isolation of affected individuals.
End.
Disclaimer: The article has not been peer-reviewed; it should not replace individual clinical judgement and the sources cited should be checked. The views expressed in this commentary represent the views of the authors and not necessarily those of the host institution, the NHS, the NIHR, the Department of Health and Social Care or the Centre for Evidence-based Medicine. The views are not a substitute for professional medical advice.
This article was review and amended June 24th 2020 with a change in the conclusion supporting further distance as opposed to a precise distance measure
AUTHORS
Zeshan Qureshi is a doctor based at St Thomas’ Hospital, and a previous Academic Clinical Fellow in Global Health.
Nick Jones is a GP and Welcome Trust Doctoral Research Fellow at the Nuffield Department of Primary Care Health Sciences, University of Oxford, UK
Robert Temple is a Medical Student at Somerville College, University of Oxford, UK
Jessica PJ Larwood is a Medical Student at St John’s College, University of Oxford, UK
Trisha Greenhalgh is Professor of Primary Care Health Sciences at the Nuffield Department of Primary Care Health Sciences, University of Oxford, UK
Lydia Bourouiba is Associated Professor at the Massachusetts Institute of Technology (MIT) and director of The Fluid Dynamics of Disease Transmission Laboratory, MIT, Cambridge, MA, USA.
SEARCH TERMS
We used the following search terms:
| (coronavirus OR covid-19) AND (transmission OR transmissability) AND (“social distancing” OR “physical distancing”) AND (1m OR 2m OR 3m OR 4m OR 5m OR 10 m OR metre OR metres OR meter OR meters) |
Google & GoogleScholar |
| (coronavirus OR covid-19) AND (“two metre rule” OR “two meter rule” OR “2 metre rule” OR “2 meter rule” OR “six feet rule” OR “six foot rule” OR “6 feet rule” OR “6 foot rule”) |
Google & GoogleScholar |
| (social distanc*[Title/Abstract] OR physical distanc*[Title/Abstract] OR workplace distanc*[Title/Abstract] OR school distanc*[Title/Abstract]) OR ((social[Title] OR physical[Title] OR work*[Title] OR school*)[Title] AND distanc*[Title]) Filters: Systematic Reviews |
PubMed |
| ((“transmission” [Subheading] OR “Disease Transmission, Infectious”[Mesh]) OR (transmiss*[Title/Abstract] OR transmit*[Title/Abstract] OR spread*[Title/Abstract])) AND (((coronavirus*[Title] OR coronovirus*[Title] OR coronoravirus*[Title] OR coronaravirus*[Title] OR corono-virus*[Title] OR corona-virus*[Title] OR “Coronavirus”[Mesh] OR “Coronavirus Infections”[Mesh] OR “Wuhan coronavirus” [Supplementary Concept] OR “Severe Acute Respiratory Syndrome Coronavirus 2″[Supplementary Concept] OR COVID-19[All Fields] OR CORVID-19[All Fields] OR “2019nCoV”[All Fields] OR “2019-nCoV”[All Fields] OR WN-CoV[All Fields] OR nCoV[All Fields] OR “SARS-CoV-2”[All Fields] OR HCoV-19[All Fields] OR “novel coronavirus”[All Fields])) AND ((social distanc*[Title/Abstract] OR physical distanc*[Title/Abstract] OR workplace distanc*[Title/Abstract] OR school distanc*[Title/Abstract]) OR ((social[Title] OR physical[Title] OR work*[Title] OR school*)[Title] AND distanc*[Title]))) |
PubMed |
| ((metre* OR meter* OR foot OR feet OR “1m” OR “2m” OR “3m” OR “4m” OR “5m” OR “3ft” OR “6ft” OR “9ft” OR “12ft” OR “15ft”) AND ((coronavirus*[Title] OR coronovirus*[Title] OR coronoravirus*[Title] OR coronaravirus*[Title] OR corono-virus*[Title] OR corona-virus*[Title] OR “Coronavirus”[Mesh] OR “Coronavirus Infections”[Mesh] OR “Wuhan coronavirus” [Supplementary Concept] OR “Severe Acute Respiratory Syndrome Coronavirus 2″[Supplementary Concept] OR COVID-19[All Fields] OR CORVID-19[All Fields] OR “2019nCoV”[All Fields] OR “2019-nCoV”[All Fields] OR WN-CoV[All Fields] OR nCoV[All Fields] OR “SARS-CoV-2”[All Fields] OR HCoV-19[All Fields] OR “novel coronavirus”[All Fields]))) AND ((social distanc*[Title/Abstract] OR physical distanc*[Title/Abstract] OR workplace distanc*[Title/Abstract] OR school distanc*[Title/Abstract]) OR ((social[Title] OR physical[Title] OR work*[Title] OR school*)[Title] AND distanc*[Title])) |
PubMed |
| ((coronavirus*[Title] OR coronovirus*[Title] OR coronoravirus*[Title] OR coronaravirus*[Title] OR corono-virus*[Title] OR corona-virus*[Title] OR “Coronavirus”[Mesh] OR “Coronavirus Infections”[Mesh] OR “Wuhan coronavirus” [Supplementary Concept] OR “Severe Acute Respiratory Syndrome Coronavirus 2″[Supplementary Concept] OR COVID-19[All Fields] OR CORVID-19[All Fields] OR “2019nCoV”[All Fields] OR “2019-nCoV”[All Fields] OR WN-CoV[All Fields] OR nCoV[All Fields] OR “SARS-CoV-2”[All Fields] OR HCoV-19[All Fields] OR “novel coronavirus”[All Fields])) AND (metre* OR meter* OR foot OR feet OR “1m” OR “2m” OR “3m” OR “4m” OR “5m” OR “3ft” OR “6ft” OR “9ft” OR “12ft” OR “15ft”) |
PubMed |
| ((((((“Influenza, Human”[Mesh]) OR “SARS Virus”[Mesh]) OR “Middle East Respiratory Syndrome Coronavirus”[Mesh]) OR “Respiratory Tract Infections”[Mesh:NoExp]) OR (respiratory[Title] OR influenza[Title] OR sars[Title] OR mers[Title])) AND ((“transmission” [Subheading] OR “Disease Transmission, Infectious”[Mesh]) OR (transmiss*[Title/Abstract] OR transmit*[Title/Abstract] OR spread*[Title/Abstract]))) AND (metre* OR meter* OR foot OR feet OR “1m” OR “2m” OR “3m” OR “4m” OR “5m” OR “3ft” OR “6ft” OR “9ft” OR “12ft” OR “15ft”) |
PubMed |
| (social distanc* OR physical distanc* OR workplace distanc* OR school distanc*) AND (transmission OR transmissability OR spread) |
LitCOVID |
| (social distanc* OR physical distanc* OR workplace distanc* OR school distanc*) AND (airborne OR droplet*) |
LitCOVID |
| (social distanc* OR physical distanc* OR workplace distanc* OR school distanc*) AND (meter* OR metre* OR feet OR foot) |
LitCOVID |
| “”(coronavirus OR covid-19) AND (distance OR distancing) AND (metre OR meter OR foot OR feet)” |
medRxiv |
| “social distancing” (match phrase words) and abstract or title “coronavirus OR covid-19” (match any words) |
medRxiv |
| title “rapid review” (match phrase words) and abstract or title “social distancing” |
medRxiv |
| ((aerosol* OR droplet*) AND (distance OR meter* OR metre* OR foot OR feet OR spread* OR dispers* OR transmi*)) AND ((coronavirus*[Title] OR coronovirus*[Title] OR coronoravirus*[Title] OR coronaravirus*[Title] OR corono-virus*[Title] OR corona-virus*[Title] OR “Coronavirus”[Mesh] OR “Coronavirus Infections”[Mesh] OR “Wuhan coronavirus” [Supplementary Concept] OR “Severe Acute Respiratory Syndrome Coronavirus 2″[Supplementary Concept] OR COVID-19[All Fields] OR CORVID-19[All Fields] OR “2019nCoV”[All Fields] OR “2019-nCoV”[All Fields] OR WN-CoV[All Fields] OR nCoV[All Fields] OR “SARS-CoV-2”[All Fields] OR HCoV-19[All Fields] OR “novel coronavirus”[All Fields])) Filters: from 2020 – 2020 |
PubMed |
| ((airbourne[Title] OR aerosol*[Title] OR droplet*[Title]) AND (distan*[Title/Abstract] OR transmi*[Title/Abstract] OR spread*[Title/Abstract] OR dispers*[Title/Abstract] OR meter*[Title/Abstract] OR metre*[Title/Abstract] OR foot[Title/Abstract] OR feet[Title/Abstract])) AND ((coronavirus*[Title] OR coronovirus*[Title] OR coronoravirus*[Title] OR coronaravirus*[Title] OR corono-virus*[Title] OR corona-virus*[Title] OR “Coronavirus”[Mesh] OR “Coronavirus Infections”[Mesh] OR “Wuhan coronavirus” [Supplementary Concept] OR “Severe Acute Respiratory Syndrome Coronavirus 2″[Supplementary Concept] OR COVID-19[All Fields] OR CORVID-19[All Fields] OR “2019nCoV”[All Fields] OR “2019-nCoV”[All Fields] OR WN-CoV[All Fields] OR nCoV[All Fields] OR “SARS-CoV-2”[All Fields] OR HCoV-19[All Fields] OR “novel coronavirus”[All Fields])) Filters: from 2020 – 2020 |
PubMed |
REFERENCES
- Papineni RS, Rosenthal FS. The Size Distribution of Droplets in the Exhaled Breath of Healthy Human Subjects. Journal of Aerosol Medicine 1997;10(2):105-16. doi: 10.1089/jam.1997.10.105
- Coronavirus disease (COVID-19) advice for the public: World Health Organisation; 2020 [Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public accessed 15/06/2020.
- Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Current Opinion in Infectious Diseases 2019;32(4):372-79. doi: 10.1097/QCO.0000000000000563
- Alagoz O, Sethi A, Patterson B, et al. Impact of Timing of and Adherence to Social Distancing Measures on COVID-19 Burden in the US: A Simulation Modeling Approach. MedRxiv 2020 doi: https://doi.org/10.1101/2020.06.07.20124859 [published Online First: 9th June, 2020]
- Du Z, Xu X, Wang L, et al. Effects of Proactive Social Distancing on COVID-19 Outbreaks in 58 Cities, China. Emerg Infect Dis 2020;26(9) doi: 10.3201/eid2609.201932 [published Online First: 10th June 2020]
- Chaw L, Koh WC, Jamaludin SA, et al. SARS-CoV-2 transmission in different settings: Analysis of cases and close contacts from the Tablighi cluster in Brunei Darussalam. medRxiv 2020:2020.05.04.20090043. doi: 10.1101/2020.05.04.20090043
- Li W, Zhang B, Lu J, et al. Characteristics of Household Transmission of COVID-19. Clinical Infectious Diseases 2020 doi: 10.1093/cid/ciaa450
- Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. The Lancet Infectious Diseases 2020 doi: 10.1016/S1473-3099(20)30287-5 [published Online First: 27 April, 2020]
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. New England Journal of Medicine 2020;382(16):1564-67. doi: 10.1056/NEJMc2004973
- UK Gov. Staying alert and Safe (Social Distancing): Government of the United Kingdom; [updated 12/06/2020. Available from: https://www.gov.uk/government/publications/staying-alert-and-safe-social-distancing/staying-alert-and-safe-social-distancing. accessed 15/06/2020.
- Bahl P, Doolan C, de Silva C, et al. Airborne or Droplet Precautions for Health Workers Treating Coronavirus Disease 2019? The Journal of Infectious Diseases 2020 doi: 10.1093/infdis/jiaa189
- Siegel JD, Rhinehart E, Jackson M, et al. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. American Journal of Infection Control 2007;35(10 Suppl 2):65. doi: 10.1016/j.ajic.2007.10.007
- Feigin RD, Baker CJ, Herwaldt LA, et al. Epidemic meningococcal disease in an elementary-school classroom. The New England Journal of Medicine 1982;307(20):1255-57. doi: 10.1056/NEJM198211113072007
- Dick EC, Jennings LC, Mink KA, et al. Aerosol transmission of rhinovirus colds. The Journal of Infectious Diseases 1987;156(3):442-48. doi: 10.1093/infdis/156.3.442
- Duguid JP. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. The Journal of Hygiene 1946;44(6):471-79.
- Bourouiba L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA 2020;323(18):1837-38. doi: 10.1001/jama.2020.4756
- Bourouiba L, Dehandschoewercker E, Bush John WM. Violent expiratory events: on coughing and sneezing. Journal of Fluid Mechanics 2014;745:537-63. doi: 10.1017/jfm.2014.88
- Bourouiba L. IMAGES IN CLINICAL MEDICINE. A Sneeze. The New England journal of medicine 2016;375(8):e15. doi: 10.1056/NEJMicm1501197
- Yu ITS, Li Y, Wong TW, et al. Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus. The New England Journal of Medicine 2004;350(17):1731-39. doi: 10.1056/NEJMoa032867
- Tang JW, Li Y, Eames I, et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. Journal of Hospital Infection 2006;64(2):100-14. doi: 10.1016/j.jhin.2006.05.022
- Wong T-w, Lee C-k, Tam W, et al. Cluster of SARS among Medical Students Exposed to Single Patient, Hong Kong. Emerging infectious diseases 2004;10(2):269-76. doi: 10.3201/eid1002.030452
- Anfinrud P, Stadnytskyi V, Bax CE, et al. Visualizing Speech-Generated Oral Fluid Droplets with Laser Light Scattering. New England Journal of Medicine 2020;382(21):2061-63. doi: 10.1056/NEJMc2007800
- Jang S, Han SH, Rhee JY. Cluster of Coronavirus Disease Associated with Fitness Dance Classes, South Korea. Emerg Infect Dis 2020;26(8) doi: 10.3201/eid2608.200633 [published Online First: 2020/05/16]
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice — Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020;69:606–10. doi: http://dx.doi.org/10.15585/mmwr.mm6919e6external
- Nishiura H, Oshitani H, Kobayashi T, et al. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). medRxiv 2020:2020.02.28.20029272. doi: 10.1101/2020.02.28.20029272
- Shim E, Tariq A, Choi W, et al. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis 2020;93:339-44. doi: 10.1016/j.ijid.2020.03.031 [published Online First: 2020/03/22]
- Leclerc QJ, Fuller NM, Knight LE, et al. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res 2020;5 doi: https://doi.org/10.12688/wellcomeopenres.15889.1
- Leung NHL, Chu DKW, Shiu EYC, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nature medicine 2020;26(5):676-80. doi: 10.1038/s41591-020-0843-2
- Gralton J, Tovey ER, McLaws ML, et al. Respiratory virus RNA is detectable in airborne and droplet particles. Journal of Medical Virology 2013;85(12):2151-59. doi: 10.1002/jmv.23698
- Henle W, Henle G. Experimental exposure of human subjects to viruses of influenza. Journal of immunology (Baltimore, Md : 1950) 1946;52:145.
- Wells WF. Airborne Contagion and Air Hygiene: An Ecological Study of Droplet Infection: . Harvard University Press 1957;38(1):65. doi: 10.1016/S0041-3879(57)80076-2
- Sonkin LS. The role of particle size in experimental air-borne infection. American Journal of Hygiene 1951;53(3):337-54. doi: 10.1093/oxfordjournals.aje.a119459
- Kim S-H, Chang SY, Sung M, et al. Extensive Viable Middle East Respiratory Syndrome (MERS) Coronavirus Contamination in Air and Surrounding Environment in MERS Isolation Wards. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2016;63(3):363-69. doi: 10.1093/cid/ciw239
- Morawska L, Cao J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environment International 2020;139:105730. doi: 10.1016/j.envint.2020.105730
- Tellier R, Li Y, Cowling BJ, et al. Recognition of aerosol transmission of infectious agents: a commentary. BMC infectious diseases 2019;19(1):101. doi: 10.1186/s12879-019-3707-y
- Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020 doi: 10.1038/s41586-020-2196-x
- Fears AC, Klimstra WB, Duprex P, et al. Comparative dynamic aerosol efficiencies of three emergent coronaviruses and the unusual persistence of SARS-CoV-2 in aerosol suspensions. medRxiv 2020:2020.04.13.20063784. doi: 10.1101/2020.04.13.20063784
- Hijnen D, Marzano AV, Eyerich K, et al. SARS-CoV-2 Transmission from Presymptomatic Meeting Attendee, Germany. Emerging infectious diseases 2020;26(8) doi: 10.3201/eid2608.201235
- Tong Z-D, Tang A, Li K-F, et al. Potential Presymptomatic Transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerging infectious diseases 2020;26(5):1052-54. doi: 10.3201/eid2605.200198
- Qian G, Yang N, Ma AHY, et al. COVID-19 Transmission Within a Family Cluster by Presymptomatic Carriers in China. Clinical Infectious Diseases 2020 doi: 10.1093/cid/ciaa316
- Anderson EL, Turnham P, Griffin JR, et al. Consideration of the Aerosol Transmission for COVID‐19 and Public Health. Risk Analysis 2020;40(5):902-07. doi: 10.1111/risa.13500
- Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility – King County, Washington, March 2020. MMWR Morbidity and mortality weekly report 2020;69(13):377-81. doi: 10.15585/mmwr.mm6913e1
- Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet 2020 doi: 10.1016/S0140-6736(20)31142-9; 0810.1016/S0140-6736(20)31142-9
- Occupational Safety and Health Administration (US). Laboratory Safety Guidance: OHSA 2011. Accessed 22nd June 2020 at https://www.osha.gov/Publications/laboratory/OSHA3404laboratory-safety-guidance.pdf.
- Park SY, Kim YM, Yi S, et al. Coronavirus Disease Outbreak in Call Center, South Korea. Emerg Infect Dis 2020;26(8) doi: 10.3201/eid2608.201274 [published Online First: 2020/04/24]
- Xu P, Qian H, Miao T, et al. Transmission routes of Covid-19 virus in the Diamond Princess Cruise ship. medRxiv 2020:2020.04.09.20059113. doi: 10.1101/2020.04.09.20059113
- Li Y, Qian H, Hang J, et al. Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. medRxiv 2020:2020.04.16.20067728. doi: 10.1101/2020.04.16.20067728
- Doung-ngern P, Suphanchaimat R, Panjangampatthana A, et al. Associations between wearing masks, washing hands, and social distancing practices, and risk of COVID-19 infection in public: a cohort-based case-control study in Thailand. MedRxiv 2020 doi: https://doi.org/10.1101/2020.06.11.20128900 [published Online First: 15 June, 2020]
- Beggs CB. Is there an airborne component to the transmission of COVID-19? : a quantitative analysis study. MedRxiv 2020 doi: https://doi.org/10.1101/2020.05.22.20109991 [published Online First: 26 May 2020]
- Burke RM, Balter S, Barnes E, et al. Enhanced Contact Investigations for Nine Early Travel-Related Cases of SARS-CoV-2 in the United States. medRxiv 2020:2020.04.27.20081901. doi: 10.1101/2020.04.27.20081901
- Cheng H-Y, Jian S-W, Liu D-P, et al. High transmissibility of COVID-19 near symptom onset. medRxiv 2020:2020.03.18.20034561. doi: 10.1101/2020.03.18.20034561
- Döhla M, Wilbring G, Schulte B, et al. SARS-CoV-2 in environmental samples of quarantined households. MedRxiv 2020 doi: https://doi.org/10.1101/2020.05.28.20114041
- Cai J, Sun W, Huang J, et al. Indirect Virus Transmission in Cluster of COVID-19 Cases, Wenzhou, China, 2020. Emerging infectious diseases 2020;26(6):1343-45. doi: 10.3201/eid2606.200412
- Yamagishi T. Environmental sampling for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during a coronavirus disease (COVID-19) outbreak aboard a commercial cruise ship. medRxiv 2020:2020.05.02.20088567. doi: 10.1101/2020.05.02.20088567
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. Jama 2020;Online communication published 26th March 2020 at https://jamanetwork.com/journals/jama/fullarticle/2763852
- Zhou J, Otter J, Price JR, et al. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. medRxiv 2020:2020.05.24.20110346. doi: 10.1101/2020.05.24.20110346
- Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020 doi: 10.1038/s41586-020-2271-3 [published Online First: 2020/04/28]
- Chia PY, Coleman KK, Tan YK, et al. Detection of Air and Surface Contamination by Severe Acute Respiratory Syndrome 2 Coronavirus 2 (SARS-CoV-2) in Hospital Rooms of Infected Patients MedRxiv 2020 doi: https://doi.org/10.1101/2020.03.29.20046557 [published Online First: April 1st, 2020]
- Ma J, Qi X, Chen H, et al. Exhaled breath is a significant source of SARS-CoV-2 emission. medRxiv 2020
- Santarpia JL, Rivera DN, Herrena V. Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center. medRxiv 2020
- Ding Z, Qian H, Xu B, et al. Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. MedRxiv 2020 doi: https://doi.org/10.1101/2020.04.03.20052175 [published Online First: April 7th, 2020]
- Ong SWX, Tan YK, Chia PY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020 doi: 10.1001/jama.2020.3227 [published Online First: 2020/03/05]
- Wu S, Wang Y, Jin X, et al. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am J Infect Control 2020 doi: 10.1016/j.ajic.2020.05.003 [published Online First: 2020/05/15]
- Guo Z-D, Wang Z-Y, Zhang S-F, et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerging infectious diseases 2020;26(7) doi: 10.3201/eid2607.200885
- Wong SCY, Kwong RTS, Wu TC, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. Journal of Hospital Infection 2020;105(2):119-27. doi: 10.1016/j.jhin.2020.03.036
- Heinzerling A, Stuckey MJ, Scheuer T, et al. Transmission of COVID-19 to Health Care Personnel During Exposures to a Hospitalized Patient – Solano County, California, February 2020. MMWR Morbidity and mortality weekly report 2020;69(15):472-76. doi: 10.15585/mmwr.mm6915e5
- Bai Y, Wang X, Huang Q, et al. SARS-CoV-2 infection in health care workers: a retrospective analysis and a model study. medRxiv 2020:2020.03.29.20047159. doi: 10.1101/2020.03.29.20047159

