What is the role of T cells in COVID-19 infection? Why immunity is about more than antibodies
October 19, 2020
Annette Plüddemann, Jeffrey K. Aronson
On behalf of the Oxford COVID-19 Evidence Service Team
Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences
University of Oxford
Pdf to download
Summary
- CD4+ T cells help B cells to produce antibodies and help CD8+ T cells to kill virus-infected cells
- One of the dominant cytokines produced by T cells is interferon gamma, a key player in controlling viral infection – see also [41]
- Lymphopenia is a main feature of COVID-19 infection, affecting CD4+ T cells, CD8+ T cells, and B cells, and is more pronounced in severely ill patients
- T cell responses in severely ill patients may be impaired, over-activated, or inappropriate, and further research is required to elucidate this and inform treatment strategies
- There is some evidence of cross-reactivity with seasonal/endemic coronaviruses
- Emerging studies suggest that all or a majority of people with COVID-19 develop a strong and broad T cell response, both CD4 and CD8, and some have a memory phenotype, which bodes well for potential longer-term immunity
- Understanding the roles of different subsets of T cells in protection or pathogenesis is crucial for preventing and treating COVID-19
What is the role of T cells and antibodies in immunity? Like B cells, which produce antibodies, T cells are central players in the immune response to viral infection [1].
When the SARS-CoV-2 virus, which causes COVID-19, infects epithelial cells, such as those found in the airways, it replicates inside the cells, using the host cell’s biochemical machinery. This causes the host cell to undergo programmed cell death, releasing molecules called damage-associated molecular patterns (e.g. nucleic acids and oligomers) [2].
These molecules are recognized by macrophages and neighbouring endothelial and epithelial cells, causing them to produce pro-inflammatory cytokines, including chemokines (Box 1); examples include
- Interleukin-6 [IL-6];
- Interferon gamma-induced protein 10 [IP-10; also known as CXCL10];
- Macrophage inflammatory protein 1α and 1β;
- Monocyte chemoattractant protein 1 [MCP-1, also known as CCL2].
Monocytes, macrophages, and T cells are then recruited to the site of infection by these chemokines and other cytokines and promote further inflammation. As part of this inflammatory response, the recruited T cells produce interferon-gamma (IFNγ) (see also [40]).
Box 1. Definitions of some of the terms used in this article

Several types of T cells are involved in this response.
CD4+ T helper (Th) cells interact with CD8+ T cells, which drive the cytotoxic response that kills cells infected with the virus. The CD8+ T cells directly recognize viral peptides presented at the surfaces of infected cells, causing apoptosis (a form of programmed cell death) and preventing the virus from spreading further.
Follicular helper T (TFH) cells are a specialized subset of CD4+ T cells that provide help to B cells through both cell-cell interactions and release of cytokines, leading to the production of antibodies by B cells [1]. These neutralizing antibodies can recognize whole viruses and act by blocking the virus from infecting cells.
Alveolar macrophages recognize the neutralized viruses and the apoptotic cells (killed by the CD8+ T cells) and clear them by phagocytosis. This then results in recovery from the viral infection (Figure 1, adapted from [2]).
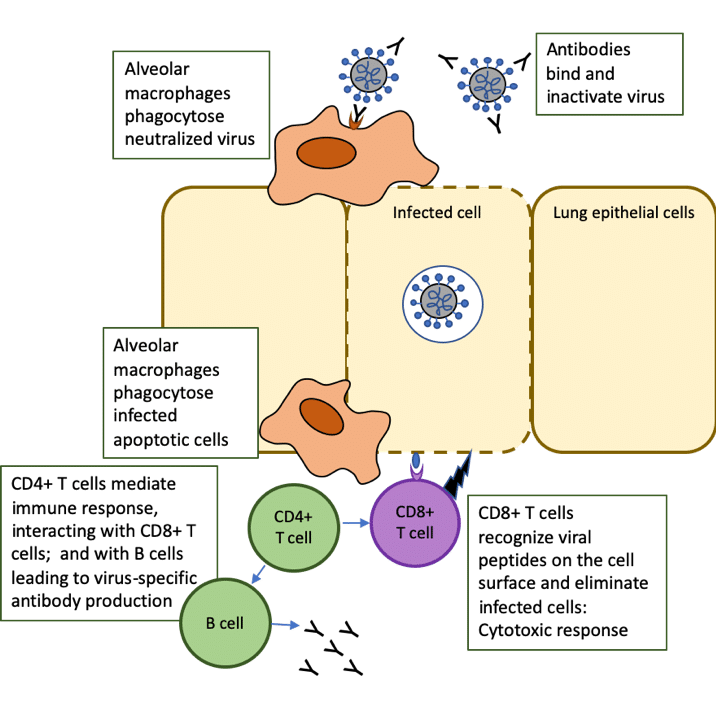
Figure 1. Role of T cells in response to COVID-19 infection: adapted from The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020 Jun;20(6):363-374. doi: 10.1038/s41577-020-0311-8.
What do we know about T cell responses and antibody production in patients with COVID-19?
Studies assessing the clinical features of patients infected with SARS-CoV-2 have reported an incubation time of 4 to 7 days before the onset of symptoms, and a further 7 to 10 days before progression to severe disease [3].
For many primary virus infections, it typically takes 7 to 10 days to prime and expand adaptive T cell immune responses to control the virus, and this correlates with the typical time it takes for patients with COVID-19 either to recover or to develop severe illness [11]. This raises the possibility that a poor initial T cell response contributes to persistence and severity of SARS-CoV-2, whereas early strong T cell responses may be protective.
Lymphopenia
One feature of SARS-CoV-2 infection, particularly in severe infection, is lymphopenia (an abnormal reduction in lymphocyte numbers), which resolves when patients recover. There are reports of a correlation between disease intensity and lymphopenia; for example, in infected children, in whom the mortality rate is very low, lymphopenia is rarely observed, while in older adults, in whom the mortality rate is higher, lymphopenia occurs more often, particularly in severe cases [12].
Depletion of CD4+ T cells, CD8+ T cells, and B cells, among other immune cells, reportedly occurs [13,14]. Although there is so far limited understanding of the mechanisms of lymphopenia in COVID-19, many patients with severe disease have reduced T cell numbers in particular, and perhaps specifically CD8+ T cells [12], but it is unclear why this is so. Lymphopenia has been reported in infections with other respiratory viruses, such as influenza [15], but seems to last longer in COVID-19 and may be more severe [14].
The CD4+ T cell response in COVID-19
Some studies have shown that in patients with severe COVID-19 there is evidence of impaired function of CD4+ T cells, including reduced IFNγ production [16], while others seem to suggest over-activation of these T cells [17].
Overall, the CD4+ T cell response in acute SARS-CoV-2 infection, whether impaired, over-activated, or inappropriate, and how this relates to disease outcomes, remains to be elucidated and is an important question. A particularly high frequency of CD4+ T cell responses specific to virus spike protein has been observed in patients who have recovered from COVID-19, which is similar to what has been reported for influenza virus infections [11]. In one small study of 14 patients, circulating virus-specific CD4+ T cells were identified in all of those who recovered from SARS- CoV-2, which also suggests the potential for developing T cell memory [18] and perhaps longer-term immunity.
The CD8+ T cell response in COVID-19
There appears to be heterogeneity in the immune response between patients. Some studies have reported that CD8+ T cells from patients with severe COVID-19 had reduced cytokine production following in vitro stimulation, and some have shown evidence of possibly exhausted T cells; in contrast, other studies have reported an overaggressive CD8+ T cell response or highly activated CD8+ T cells with increased cytotoxic response in patients with COVID-19 [14].
It is still unclear how the heterogeneity of the CD8+ T cell response relates to disease features, which could be driven by, for example, patient immunotypes [17,19] or the nature of the interaction between respiratory epithelial cells and cytotoxic T cells and the level of response.
Several chemokine receptor genes (including CCR9, CXCR6, and XCR1) and the locus controlling the ABO blood type have been identified as being associated with severe disease; however, whether these genes are directly or indirectly related to T cell responses in COVID-19 remains unknown [14].
A higher proportion of CD8+ T cell responses was observed in patients who only developed mild disease, suggesting a potential protective role of CD8+ T cell responses [11]. Most of the CD8+ T cell responses were specific to viral internal proteins, rather than spike proteins, which should be considered in vaccine development [4]. SARS-CoV-2-specific CD8+ T cells are present in about 70% of patients who have recovered [18], which is evidence of a virus-specific CD8+ T cell response and the presence of CD8+ T cell memory. However, the ability of these cells to protect from future infection remains to be determined.
Potential for cross-reactive immunity
In one study, SARS-CoV-2-reactive CD4+ T cells were also identified in about 40 to 60% of unexposed individuals, suggesting cross-reactive T cell recognition between circulating ‘‘common cold’’ coronaviruses and SARS-CoV-2 [18]. Another study also showed cross-reactive memory T cells in patients who had recovered from SARS-CoV 17 years before (n=23), and also in individuals with no history of SARS infection (n=37) [20]. These studies were done in small numbers of patients and need verification.
Potential for long-term immunity
Early research suggests that the antibodies in people infected with SARS-CoV-2 dropped significantly within 2 to 3 months [21,22], causing concern that humoral immunity against the virus may decline rapidly. However, it is a normal part of the immune response that antibody levels fall after an infection has resolved [23]. For example, in seasonal coronavirus infections, antibodies start to decline at about a week after infection and typically only last for about a year [24]. It should also be noted that memory T and B cells are formed after infection [25,26]; these can be reactivated when another infection with the same virus occurs and could provide long-lasting immunity. A preliminary study that has not yet undergone peer review has shown that memory T and B cells were found in patients with mild COVID-19 symptoms who had recovered and that these cells persisted, suggesting the potential for longer-term immunity [27].
SARS-CoV-2-specific memory T cells have also been detected in exposed seronegative healthy individuals (relatives of confirmed cases), which may indicate asymptomatic infection. One study has shown that ~93% of “exposed asymptomatic” individuals had a T cell response to SARS-CoV-2, despite seropositivity in only 60% of cases [28]. Asymptomatic infections may therefore be more common, and antibody testing alone may underestimate the true prevalence of the infection or population immunity. SARS-CoV-2-specific T cells were found in most of the convalescent patients in this study, which is a promising sign that infection may give rise to immunity [29]
Potential therapeutic interventions
Interleukin 7 (IL-7), a cytokine that is essential for lymphocyte survival and expansion, may provide a promising therapeutic strategy and when given to patients can increase circulating and tissue lymphocytes [30]. However, randomized controlled trials are required to assess the safety and efficacy of IL-7 as a treatment, and some are currently underway [31]. A clearer understanding of the immune response at different stages of disease and differences in immune response between patients could help inform the use of immunostimulatory strategies such as thymosin α1 [32] or type I interferon [33] versus immunosuppressive drugs such as tocilizumab [34], ruxolitinib [35], or dexamethasone [36] to treat COVID-19.
Questions remain around the use of immune checkpoint inhibitors, for example, in cancer therapy, and their role in COVID-19 infection. An example of these are inhibitors of programmed death-1/ligand-1 (PD-1/PD-L1) (e.g. nivolumab and pembrolizumab). COVID-19 may cause T-cell exhaustion with increased expression of PD-1 and PD-L1, and the effect of blockade of these critical pathways is unknown. It could theoretically either mitigate or exacerbate COVID-19 severity [37], depending on the stage of the disease. Trials are required to evaluate these interventions in COVID-19; one trial evaluating pembrolizumab as part of a study assessing checkpoint blockade interventions in COVID-19 is currently underway [38] and several trials are registered planning to assess nivolumab safety and efficacy in patients with COVID-19 [39]. In one study of the effect of PD-1 blockade on the severity of COVID-19 in patients with lung cancers, PD-1 blockade did not appear to affect the severity of COVID-19 in patients with lung cancers [40].
References
- Swain S, McKinstry KK, Strutt TM. Expanding roles for CD4⁺ T cells in immunity to viruses. Nat Rev Immunol 2012; 12(2): 136-48.
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20(6): 363-74.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223): 497-506. [Erratum in Lancet 2020; 395(10223): 496.]
- Dumonde DC, Wolstencroft RA, Panayi GS, Matthew M, Morley J, Howson WT “Lymphokines”: non-antibody mediators of cellular immunity generated by lymphocyte activation. Nature 1969; 224(5214): 38-42.
- Bigazzi PE, Yoshida T, Ward PA, Cohen S. Production of lymphokine-like factors (cytokines) by simian virus 40-infected and simian virus 40-transformed cells. Am J Pathol 1975; 80(1): 69-78.
- IUPHAR/BPS Guide to Pharmacology. Chemokine receptors. https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=14.
- Coperchini F, Chiovato L, Croce L, Magri F, Rotondia M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 2020; 53: 25–32.
- IUIS-WHO Nomenclature Subcommittee. Nomenclature for clusters of differentiation (CD) of antigens defined on human leukocyte populations. Bull World Health Organ 1984; 62(5): 809-15.
- Beare A, Stockinger H, Zola H, Nicholson I. Monoclonal antibodies to human cell surface antigens. Curr Protoc Immunol 2008; 80(1): A.4A.1–A.4A.73.
- Durand PM, Ramsey G. The nature of programmed cell death. Biol Theory 2019; 14: 30–41.
- Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19 [published online ahead of print, 2020 Sep 4]. Nat Immunol 2020; 10.1038/s41590-020-0782-6.
- Tavakolpour S, Rakhshandehroo T, Wei EX, Rashidian M. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett 2020; 225: 31-2.
- Zhou R, To KK, Wong YC, et al. Acute SARS-CoV-2 infection impairs dendritic cell and T Cell responses. Immunity 2020; S1074-7613(20)30333-2.
- Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol 2020; 20(9): 529-36.
- McClain MT, Park LP, Nicholson B, et al. Longitudinal analysis of leukocyte differentials in peripheral blood of patients with acute respiratory viral infections. J Clin Virol 2013; 58(4): 689-95.
- Sattler A, Angermair S, Stockmann H, et al. SARS-CoV-2 specific T-cell responses and correlations with COVID-19 patient predisposition. J Clin Invest 2020; 140965.
- Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals patient heterogeneity and distinct immunotypes with implications for therapeutic interventions. Preprint. bioRxiv 2020; 2020.05.20.106401. Published 2020 May 23.
- Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020; 181(7): 1489-501.e15.
- Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med 2020; NEJMoa2020283.
- Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584(7821): 457-62.
- Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26(8): 1200-4.
- Seow J, Graham C, Merrick B, et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. https://www.medrxiv.org/content/10.1101/2020.07.09.20148429v1 (Accessed 10/09/2020). Preprint, not peer-reviewed.
- Ledford H. What the immune response to the coronavirus says about the prospects for a vaccine. Nature 2020; 585: 20-1.
- Callow KA, Parry HF, Sergeant M, Tyrrell DA. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect 1990; 105(2): 435-46.
- Akkaya M, Kwak K, Pierce SK. B cell memory: building two walls of protection against pathogens. Nat Rev Immunol 2020; 20(4): 229-38.
- Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol 2016; 16(2): 90-101.
- Rodda L, Jason N, Shehata L, et al. Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. https://www.medrxiv.org/content/10.1101/2020.08.11.20171843v2. (Accessed 10/09/2020). Preprint, not peer-reviewed.
- Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020. doi: https://doi.org/10.1016/j.cell.2020.08.017. Journal pre-proof.
- Cañete PF, Vinuesa CG. COVID-19 makes B cells forget, but T cells remember. Cell 2020. doi: https://doi.org/10.1016/j.cell.2020.09.013. Journal pre-proof.
- Laterre PF, François B, Collienne C, Hantson P, Jeannet R, Remy KE, Hotchkiss RS. Association of interleukin 7 immunotherapy with lymphocyte counts among patients with severe coronavirus disease 2019 (COVID-19). JAMA Netw Open 2020; 3(7): e2016485. Published 2020 Jul 1
- US National Library of Medicine. Interleukin 7 ǀ https://clinicaltrials.gov/ct2/results?cond=Covid19&term=IL-7&cntry=&state=&city=&dist.
- US National Library of Medicine. Thymosin Apha 1 ǀ https://clinicaltrials.gov/ct2/results?cond=COVID&term=Thymosin+Alpha+1&cntry=&state=&city=&dist=
- US National Library of Medicine. Type 1 Interferon ǀ https://clinicaltrials.gov/ct2/results?cond=COVID&term=type+1+interferon&cntry=&state=&city=&dist=
- US National Library of Medicine. Tocilizumab ǀ https://clinicaltrials.gov/ct2/results?cond=COVID&term=tocilizumab&cntry=&state=&city=&dist=
- US National Library of Medicine. Ruxolitinib ǀ https://clinicaltrials.gov/ct2/results?cond=COVID&term=ruxolitinib&cntry=&state=&city=&dist=
- US National Library of Medicine. Dexamethasone ǀ https://clinicaltrials.gov/ct2/results?cond=COVID&term=dexamethasone&cntry=&state=&city=&dist=
- Sullivan RJ, Johnson DB, Rini BI, Neilan TG, Lovly CM, Moslehi JJ, Reynolds KL. COVID-19 and immune checkpoint inhibitors: initial considerations. J Immunother Cancer 2020; 8(1): e000933
- US National Library of Medicine. Pembrolizumab ǀ https://clinicaltrials.gov/ct2/results?cond=COVID&term=Pembrolizumab&cntry=&state=&city=&dist=
- US National Library of Medicine. Nivolumab ǀ https://clinicaltrials.gov/ct2/results?cond=COVID&term=nivolumab&cntry=&state=&city=&dist=
- Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov 2020; 10(8): 1121-8.
- Aronson JK, DeVito N, Plüddeman A, Ferner RE. Drug Vignettes: Interferons. https://www.cebm.net/covid-19/drug-vignettes-interferons/
Acknowledgements: The authors would like to thank Dr. T. Griseri for helpful discussions.
Disclaimer: This article has not been peer-reviewed; it should not replace individual clinical judgement, and the sources cited should be checked. The views expressed in this commentary represent the views of the authors and not necessarily those of the host institution, the NHS, the NIHR, or the Department of Health and Social Care. The views are not a substitute for professional medical advice.

